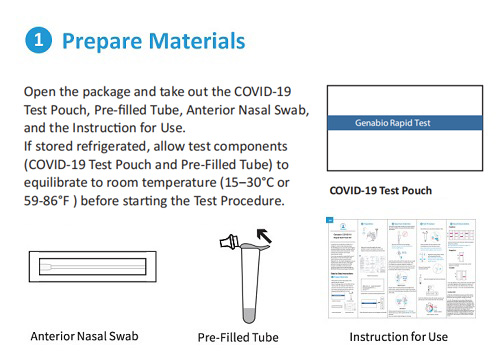
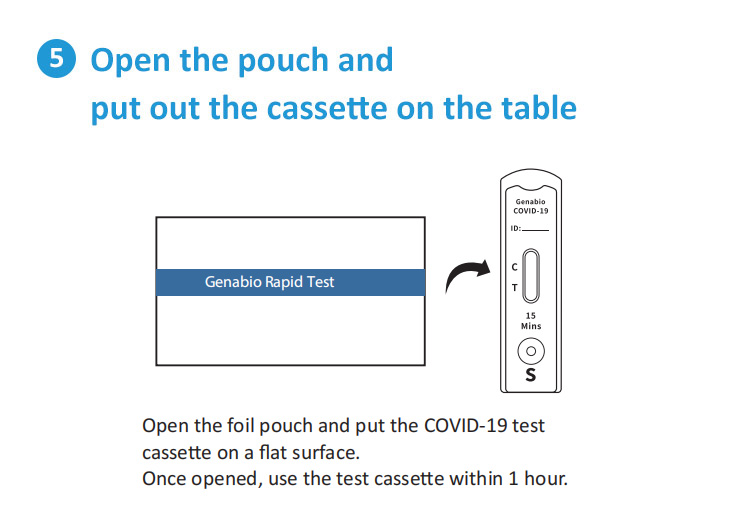
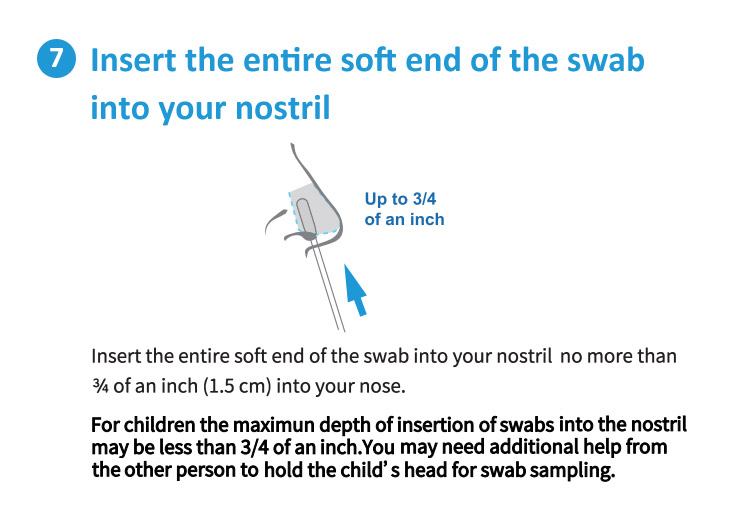
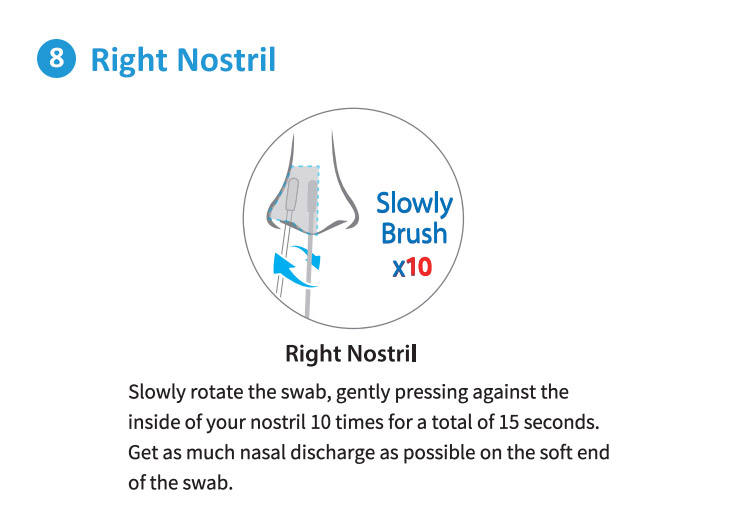
Each Box Contains 2 Tests.
Genabio COVID-19 Rapid Self-Test is easy to use and give you accurate rapid results in as few as 15 minutes in the comfort of your own home. This test is for over-the-counter (OTC) at home and other non-laboratory sites for patients 2 years or older who are suspected of COVID-19 by a healthcare provider within the first 7 days of symptom onset. This test is also for nonprescription or over-the-counter (OTC) at home and other non-laboratory sites for screening of individuals 2 years or older without symptoms or other reasons to suspect COVID-19 infection when tested twice over three days with at least 24 hours and no more than 48 hours between tests.
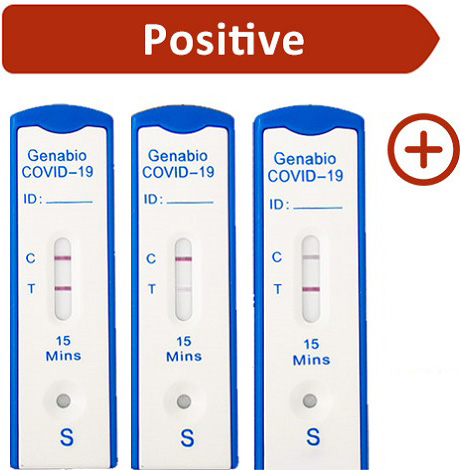
Control (C) line and Test (T) line both appear as pink-colored lines in the show window.
Note: Any faint visible pink color Test (T) line should be interpreted as positive, when the Control (C) line is also present. The Test (T) line may vary in shade an intensity (light or dark, weak or strong) depending on the concentration of antigen present in the sample. The intensity of the Control (C) line should not be compared to that of the Test (T) line for interpretation of the test result.
You do not need to perform repeat testing if you have a positive result at any time.
A positive test result means that the virus that causes COVID-19 was detected in your sample and it is very likely you have COVID-19 and are contagious. Please contact your doctor/primary care physician or your local health authority immediately and adhere to the local guidelines regarding self-isolation. There is a very small chance that this test can give a positive result that is incorrect (a false positive). Your healthcare provider will work with you to determine how best to care for you based on your test results along with medical history and your symptoms.
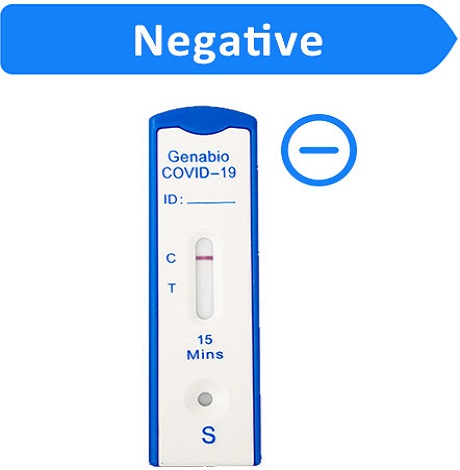
If the Control (C) line is visible, but the Test (T) line is not visible, the test is negative.
To increase the chance that the negative result for COVID-19 is accurate, you should:
•Test again in 48 hours if you have symptoms on the first day of testing.
•Test 2 more times at least 48 hours apart if you do not have symptoms on the first day of testing.
A negative test result indicates that the virus that causes COVID-19 was not detected in your sample. A negative result is presumptive, meaning it is not certain that you do not have COVID-19. You may still have COVID-19 and you may still be contagious. There is a higher chance of false negative results with antigen tests compared to laboratory-based tests such as PCR. If you test negative and continue to experience COVID-19-like symptoms, (e.g., fever, cough, and/or shortness of breath) you should seek follow up care with your health care provider.
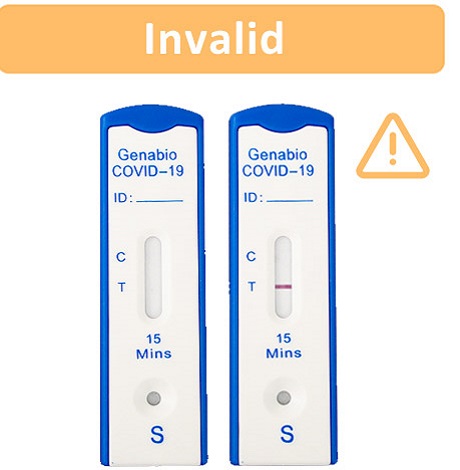
If no line appears in the Control (C) area, the test results are invalid regardless of the presence or absence of a line in the Test (T) area. An invalid result means the test was not able to tell if you have COVID-19 or not. If the test is invalid, re-test with a new swab and new test device.
Report your test result(s) at Genabio.com under "Report Test Results" – this voluntary reporting helps public health teams understand COVID-19 spread in your area and across the country and informs public health decisions.
As always, we remain available for assistance with any question, comments or to report issues with the Genabio Self Tests. Please contact us at 914 664 7500 or email us at info@bonafidemasks.com.